
The automotive industry is increasingly unified in its belief that electric propulsion will dominate the future of land transportation. Although electric mobility has regained popularity—reminding us that internal combustion engines and electric cars coexisted in the early automotive era—a significant discussion is emerging regarding the most effective means of powering electric motors. Currently, battery packs in vehicles are the preferred option, but hydrogen fuel cells present an alternative.
 Honda Clarity Fuel Cell Vehicle
Honda Clarity Fuel Cell VehicleWhat is a Fuel Cell?
In light of the limitations of contemporary battery technology, some industry experts advocate for a different approach: generating electricity onboard the vehicle without the need for heavy, cumbersome batteries. This technology is not new; it was first employed by NASA in the 1960s to generate electricity during the Gemini and Apollo missions to the Moon. While various types of fuel cells exist, proton exchange membrane fuel cells (PEMFC) are nearly exclusively used in the automotive sector.
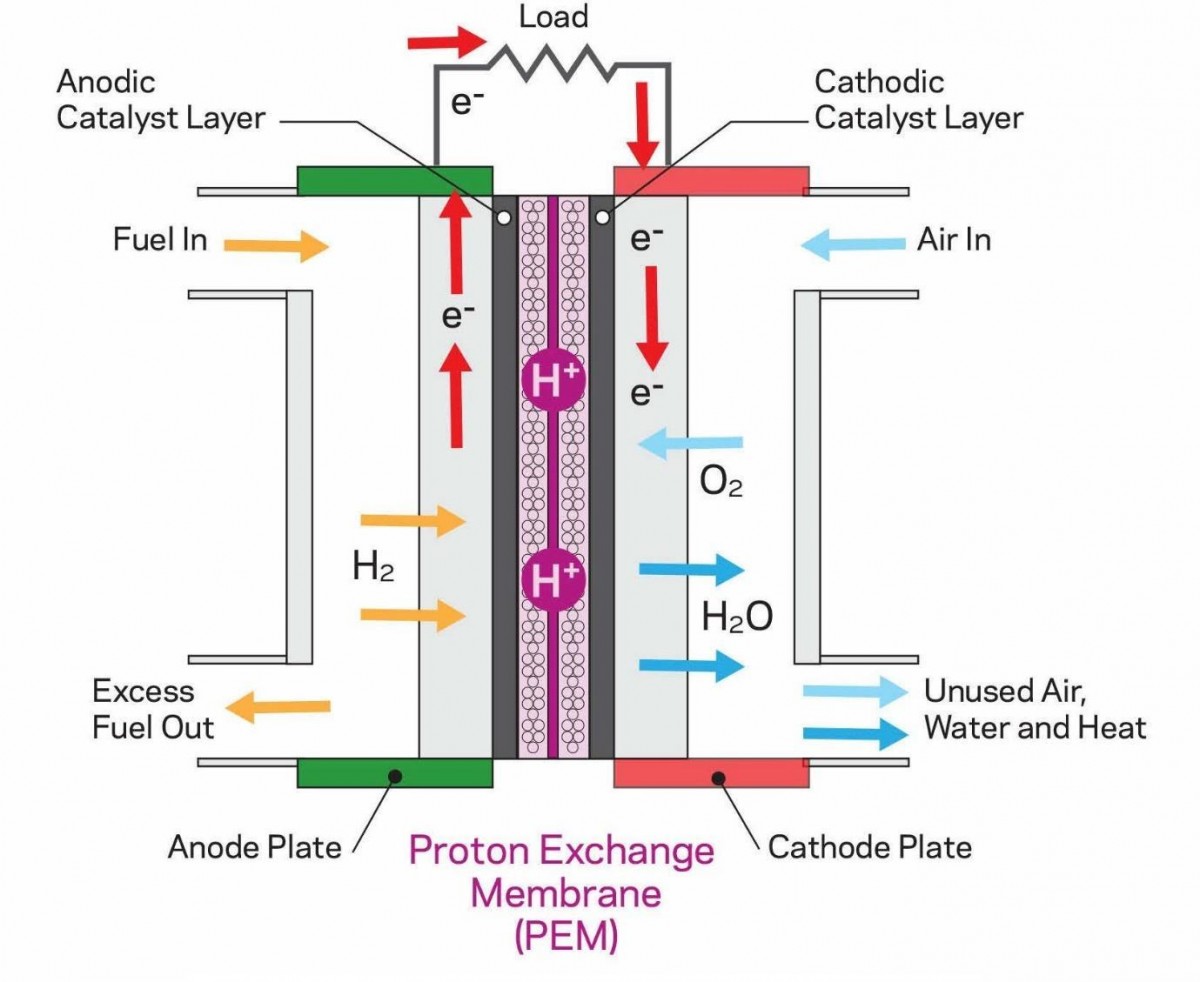
How does a fuel cell generate electricity? The process is relatively straightforward. A fuel cell consists of three main components: the anode, the cathode, and the separator. Hydrogen (H2) from a tank is introduced at the anode. H2 comprises two electrons and two protons; the positively charged protons pass through the separator while the negatively charged electrons flow through an external circuit, thus generating an electric current.
At the cathode, atmospheric oxygen encounters the protons from the hydrogen—having already traversed the separator—and the electrons from the external circuit. This interaction results in the formation of water, which is expelled from the vehicle's tailpipe. Remarkably, this is water that can be consumed!
 Nafion Proton Exchange Membrane Fuel Cell
Nafion Proton Exchange Membrane Fuel CellHydrogen Production Methods
Hydrogen is the most abundant element in the universe, but it does not exist in a free form on Earth, necessitating various extraction methods. Currently, there are four primary approaches to produce hydrogen.
The first, known as gray hydrogen, is produced through steam reforming of natural gas, where natural gas is subjected to high-temperature, high-pressure steam. While this method generates H2 and CO2, it is the least environmentally friendly.
Blue hydrogen follows a similar process but incorporates carbon capture and storage technologies to prevent CO2 emissions from entering the atmosphere.
The third method, electrolysis, yields green hydrogen. This process operates in reverse to the fuel cell system, using electric current to split water into its constituent elements—hydrogen and oxygen.
The emerging method, called turquoise hydrogen, involves passing natural gas through molten metal, resulting in hydrogen and carbon. Although promising, this technology is not yet ready for widespread implementation.
Among these methods, green hydrogen holds the most potential for sustainable and environmentally friendly production, but this hinges on generating the required electricity from renewable sources like wind or solar energy.
 Hydrogen Production through Sustainable Electrolysis
Hydrogen Production through Sustainable ElectrolysisStates of Hydrogen
Hydrogen can be utilized in two forms: gaseous and liquid, each with its own advantages and disadvantages.
Due to its status as the least dense element in the universe, hydrogen must be significantly compressed for use in vehicles. In current applications, this compression reaches up to 700 bars, requiring specialized and costly tanks.
Conversely, when in liquid form, hydrogen must be cooled to within 20°C of absolute zero—approximately -253°C. Thus, energy is necessary either for high-pressure compression or for extreme cooling.
Despite these challenges, the benefits of using hydrogen are substantial. One kilogram of hydrogen can provide 33.3 kWh of energy, equating to 167 times more energy per kilogram than the most advanced battery packs currently available.
 Toyota Mirai’s Gaseous H2 Tanks are Made from Polyamide and Carbon Fiber
Toyota Mirai’s Gaseous H2 Tanks are Made from Polyamide and Carbon FiberComparing Batteries and Hydrogen
Which option is preferable: carrying electricity or generating it onboard? Presently, the automotive industry favors battery packs for several reasons.
Primarily, the efficiency of PEMFC is approximately 60%, compared to 70% for steam reforming of natural gas and 75% for electrolysis. Hydrogen compression achieves around 89% efficiency, while liquefaction achieves approximately 66% efficiency. The following table summarizes and compares losses between battery packs and hydrogen, illustrating the most efficient scenarios for hydrogen in use:
| Efficiency Comparison | Hydrogen | Battery Pack |
|---|---|---|
| Electrolysis (%) | 75 | - |
| Hydrogen Compression (%) | 89 | - |
| Fuel/Grid Transport (%) | 80 | 93 |
| Fuel Cell (%) | 60 | 94 |
| Permanent Magnet Motor (%) | 94 | 94 |
| DC/AC Conversion (%) | 93 | 93 |
| Charging (%) | - | 94 |
| Transmission (%) | 95 | 95 |
| Total Efficiency (%) | 27 | 73 |
The data suggests that the fuel cell solution offers efficiency nearly equivalent to that of a traditional internal combustion engine (ICE) vehicle. Given the current context, fuel cells are not yet practical for passenger vehicles, leading most manufacturers to favor battery packs despite their inherent limitations.
Furthermore, it should be noted that refueling a hydrogen vehicle costs about 7 to 8 times more than recharging an electric car at home. Hydrogen could find potential applications in heavy-duty trucks due to its high energy density, provided sustainable production methods are developed.





